acetic acid and sodium hydroxide balanced equation|How to Write the Net Ionic Equation for Acetic acid + Sodium : Tagatay CH 3 COOH + NaOH → NaCH 3 COO + H2O Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Tingnan ang higit pa Note : Ici, vous contactez l'équipe du site, pas les teams de scantrad
acetic acid and sodium hydroxide balanced equation,Balanced Chemical Equation. CH 3 COOH + NaOH → NaCH 3 COO + H 2 O. Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information. Word Equation. Acetic Acid + Sodium Hydroxide = Sodium Acetate + Water. Tingnan ang higit pa
CH 3 COOH + NaOH → NaCH 3 COO + H2O Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Tingnan ang higit pa

To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation . Tingnan ang higit paWord Equation CH3COOH + NaOH = NaCH3COO + H2O is a Double Displacement (Metathesis) reaction where one mole of Acetic Acid [CH 3 COOH] and one mole of Sodium Hydroxide [NaOH] react to form one mole of Sodium Acetate [NaCH 3 . Tingnan ang higit pa
Read our article on how to balance chemical equations or ask for help in our chat. Balance any equation or reaction using this . Tingnan ang higit paHun 8, 2017 — Acetic acid, "CH"_3"COOH", will react with sodium hydroxide, "NaOH", to produce sodium acetate, "CH"_3"COONa", and water. The unbalanced chemical .
Balanced equation for acetic acid CH 3 COOH with Sodium hydroxide NaOH. When Acetic acid CH 3 COOH reacts with Sodium hydroxide NaOH, the formation of S odium acetate .
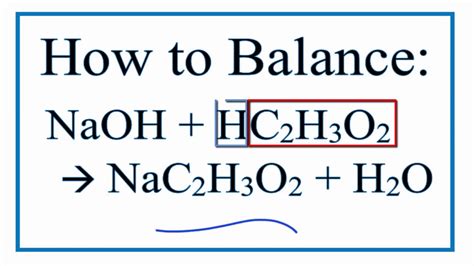
How to Write the Net Ionic Equation for Acetic acid + Sodium Balanced Chemical Equation. HC 2 H 3 O 2 + NaOH → H 2 O + NaC 2 H 3 O 2. Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by-step answer, or .Set 22, 2021 — Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. The Molarity of Acetic Acid in Vinegar. Use your two best sets of results (with the palest .The reaction of Sodium hydroxide and Acetic acid (also called Ethanoic acid) represents a net ionic equation involving a strong base and a weak acid. Strong bases are .
Okt 21, 2019 — There are three main steps for writing the net ionic equation for Acetic acid and Sodium hydroxide. First, we balance the molecular equation. Second, we writ.
In the case of acetic acid and sodium hydroxide, the reactants are CH 3 COOH and NaOH, while the products are water (H 2 O) and sodium acetate (CH 3 COONa). Next, we .According to the Arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce H + ions (protons; Equation \(\PageIndex{1}\) ), and a base is a substance like sodium .Ene 19, 2018 — Explanation: acetic acid (ethanoic acid): CH 3COOH. CH 3COOH = C2H 4O2. sodium hydroxide: N aOH. neutralisation reactions always produce a salt and .Set 16, 2022 — Example \(\PageIndex{1}\): Propionic Acid + Calcium Hydroxide. Calcium propionate is used to inhibit the growth of molds in foods, tobacco, and some medicines. Write a balanced chemical equation for the reaction of aqueous propionic acid (CH 3 CH 2 CO 2 H) with aqueous calcium hydroxide [Ca(OH) 2].

Okt 23, 2023 — A mixture of acetic acid and sodium acetate is acidic because the K a of acetic acid is greater than the K b of its conjugate base acetate. It is a buffer because it contains both the weak acid and its salt. . If we add a base such as sodium hydroxide, the hydroxide ions react with the few hydronium ions present. . [acid] = 100\): In .The balanced equation for the reaction between acetic acid and sodium hydroxide is shown below: HC2H3O2(aq) + NaOH(aq) à H2O(l) + Na C2H3O2(aq) Suppose that you perform a titration experiment to determine the concentration of acetic acid in a sample of vinegar, like we did in our lab experiment. The titrant is a standardized solution of 0.4755 .
Abr 28, 2021 — Neutralization reactions. Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt, in chemistry, is any ionic compound made by combining an acid with a base.A reaction between an acid and a base is called a neutralization reaction and can be represented as:. acid + base .Net ionic equations are a way to represent chemical reactions by showing only the species that participate in the reaction, ignoring spectator ions. This simplification allows us to focus on the essential components of the reaction. For example, when acetic acid (CH 3 COOH) reacts with sodium hydroxide (NaOH), the complete ionic equation would include all .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Ene 29, 2024 — Acetic acid, HC 2 H 3 O 2, is a weak acid. Think of the acid molecules as “potential” H + and C 2 H 3 O 2 – ions, however, these “potential” ions are held together by a covalent bond. A small percentage of the acid molecules do actually ionize (break apart into ions) when they dissolve in water, but most of the weak acid molecules do .A solution containing appreciable amounts of a weak conjugate acid-base pair is called a buffer solution, or a buffer.Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 14.14).A solution of acetic acid and sodium acetate (CH 3 COOH + CH 3 COONa) is an example of a buffer that consists of .
Ago 30, 2022 — To find how much OH-will be in excess we subtract the amount of acid and hydroxide. mmoles of hydroxide in excess: 7.8 mmol - 7.50 mmol= 0.3 mmol OH-To find the concentration of the OH-we must divide by the total volume. This is the initial volume of HF, 25 mL, and the addition of NaOH, 26 mL. Therefore the total volume is 25 mL + 26 .
Write the molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between sodium hydroxide and acetic acid. Write the balanced equation for the neutralization of acetic acid by sodium hydroxide. Be sure to use subscripts correctly and include all phase labels.Set 12, 2022 — For example, 1 L of a solution that is 1.0 M in acetic acid and 1.0 M in sodium acetate has a greater buffer capacity than 1 L of a solution that is 0.10 M in acetic acid and 0.10 M in sodium acetate even though both solutions have the same pH. The first solution has more buffer capacity because it contains more acetic acid and acetate ion.acetic acid and sodium hydroxide balanced equation How to Write the Net Ionic Equation for Acetic acid + Sodium Question: 1. Write the complete balanced equation for the neutralization reaction of acetic acid and sodium hydroxide. 2. Calculate the equivalence point for this titration. Analyte solution: 25 mL of 0.1M acetic acid. Titrant: 0.1 M sodium hydroxide.
Figure \(\PageIndex{1}\): A neutralization reaction takes place between citric acid in lemons or acetic acid in vinegar, and the bases in the flesh of fish. Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution.Ene 19, 2018 — C_2H_4O_2 + NaOH = C_2H_3O_2Na + H_2O acetic acid (ethanoic acid): CH_3COOH CH_3COOH = C_2H_4O_2 sodium hydroxide: NaOH neutralisation reactions always produce a salt and water. in this case, the salt is sodium ethanoate (CH_3COONa, C_2H_3O_2Na) equation: C_2H_4O_2 + NaOH = C_2H_3O_2Na + .
acetic acid and sodium hydroxide balanced equationThe balanced equation will be calculated along with the solubility states, complete ionic equation, net ionic equation, spectator ions and precipitates. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, .
Peb 20, 2021 — Vinegar is a complex mixture that contains acetic acid as its acidic component. Using a vinegar sample, you will determine the precise concentration of an acetic acid (CH 3 COOH) solution by titration. Because acids will react with bases, you will use a solution of sodium hydroxide (NaOH).
acetic acid and sodium hydroxide balanced equation|How to Write the Net Ionic Equation for Acetic acid + Sodium
PH0 · Write the balanced chemical equation of the reaction between ace
PH1 · What is the balanced equation for the reaction between acetic acid and
PH2 · What is the balanced equation for the reaction between acetic
PH3 · What is the balanced equation for the reaction between
PH4 · Net Ionic Equation for NaOH + CH3COOH (Strong Base and
PH5 · How to Write the Net Ionic Equation for Acetic acid + Sodium
PH6 · How do you write the balanced neutralization equation for the reaction
PH7 · How do you write the balanced neutralization equation for
PH8 · HC2H3O2 + NaOH = H2O + NaC2H3O2
PH9 · CH3COOH + NaOH = NaCH3COO + H2O
PH10 · Balancing A Net Ionic Equation For Acetic Acid And Sodium Hydroxide
PH11 · Balancing A Net Ionic Equation For Acetic Acid And Sodium
PH12 · 4.7: Acid Base Reactions
PH13 · 11: Titration of Vinegar (Experiment)